Backed by rigorous clinical trials, Addyi has been evaluated in thousands of women to establish its safety and effectiveness in treating hypoactive sexual desire disorder (HSDD).
real science, real results.

real science, real results.
Backed by rigorous clinical trials, Addyi has been evaluated in thousands of women to establish its safety and effectiveness in treating hypoactive sexual desire disorder (HSDD).

The efficacy of Addyi was established in three 24-week, randomized, double-blind, placebo-controlled trials in premenopausal women with acquired, generalized HSDD. Participants were age 19-55 years (mean 36 years) with an average HSDD and relationship duration of 5 years and 11 years, respectively. Approximately 40% subjects were also taking hormonal contraceptives. Women in these trials were treated with Addyi 100mg (n=1187) once-daily at bedtime, or placebo (n=1188).
| Study 1 | Study 2 | Study 3 | |
|---|---|---|---|
| ADDYI (n=280) Placebo (n=290) |
ADDYI (n=365) Placebo (n=372) |
ADDYI (n=532) Placebo (n=536) |
|
| Co-primary Endpoints | SSEs eDiary Desire |
SSEs eDiary Desire |
SSEs FSFI-D |
| Secondary Endpoints | FSFI-D FSDS-R Q13 |
FSFI-D FSDS-R Q13 |
FSDS-R Q13 |
SSEs = satisfying sexual events; FSFI-D = Female Sexual Function Index – Desire Domain; FSDS-R Q13 = Female Sexual Distress Scale-Revised Question 13
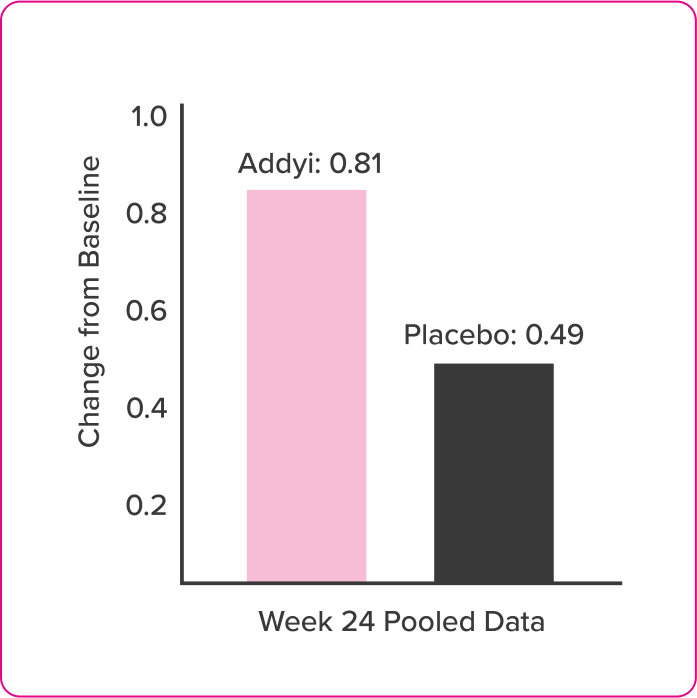
The efficacy of Addyi for the treatment of acquired, generalized HSDD in naturally postmenopausal women less than 65 years of age was established in a single 24-week, randomized, double-blind, placebo-controlled trial. The trial included postmenopausal women with acquired, generalized HSDD of at least 6 months duration. The trial had two co-primary efficacy endpoints – one for SSEs counts and one for desire domain of the Female Sexual Function Index (FSFI Desire) – and a secondary endpoint that measured bother (a component of distress) related to sexual desire using Question 13 FSDS-R. The patients were treated with Addyi 100mg once daily at bedtime (n = 430) or placebo (n = 451).
Addyi resulted in statistically significant improvement compared to placebo in the co-primary and secondary endpoints.
| Study 1 | |
|---|---|
| ADDYI (n=430) Placebo (n=451) |
|
| Co-primary Endpoints | SSEs FSFI-D |
| Secondary Endpoints | FSDS-R Q13 |
In clinical trials, all three end points were met.
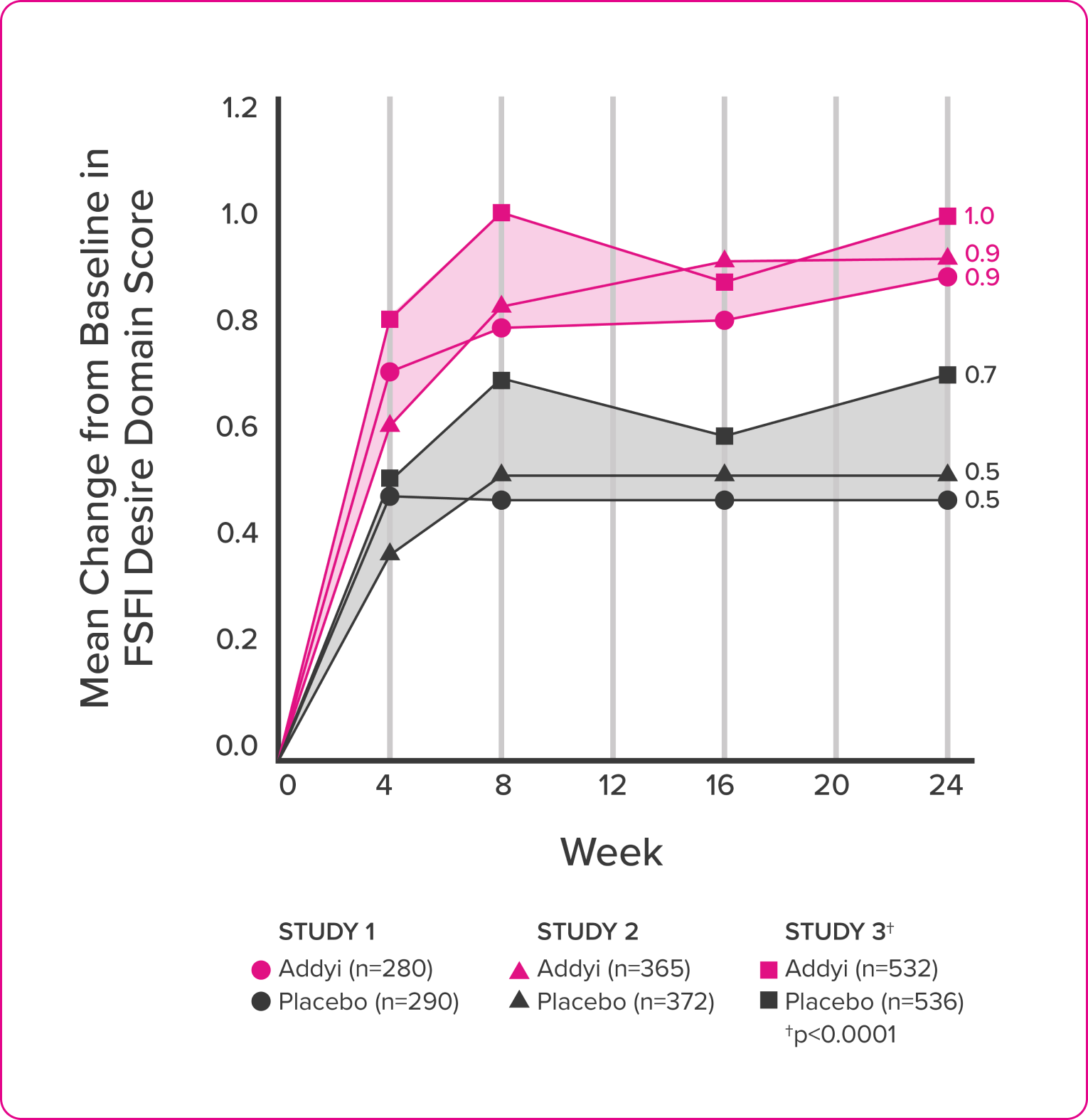
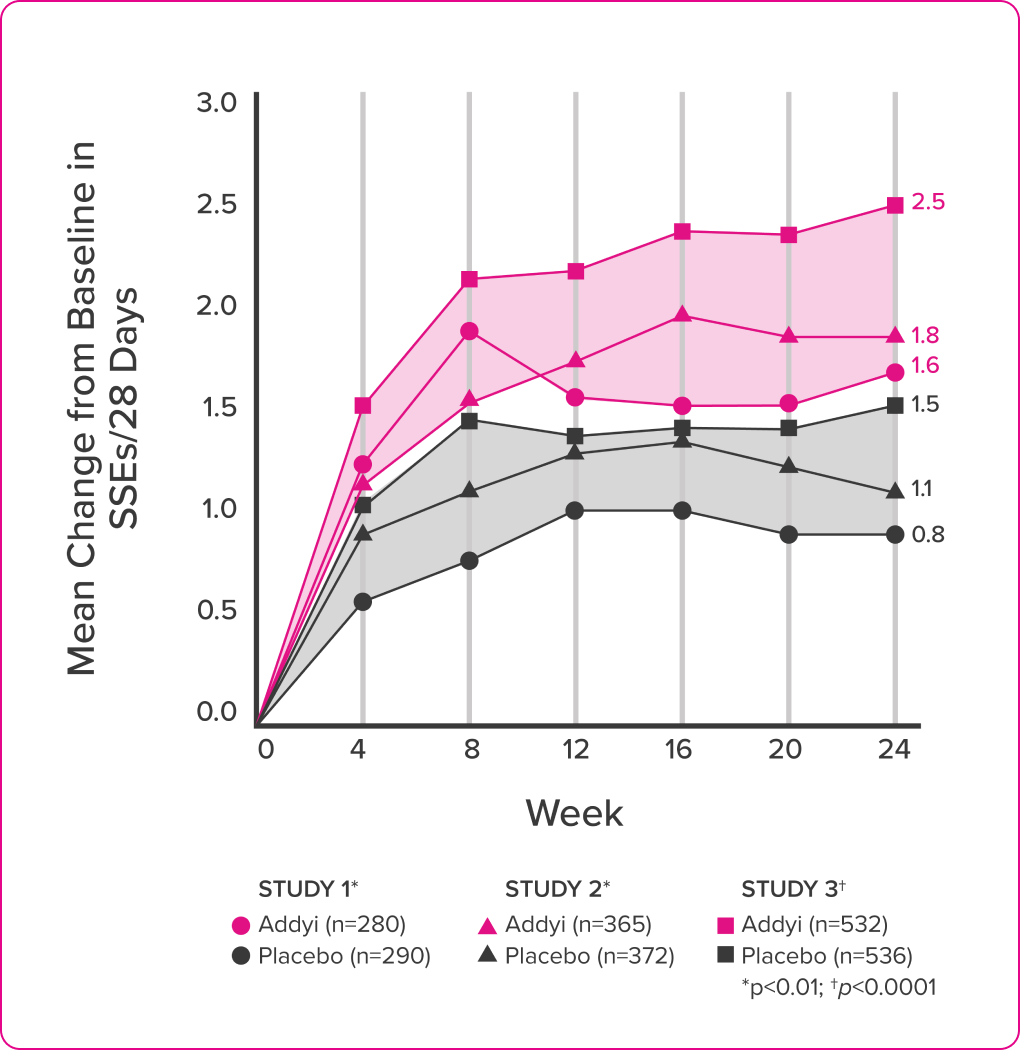
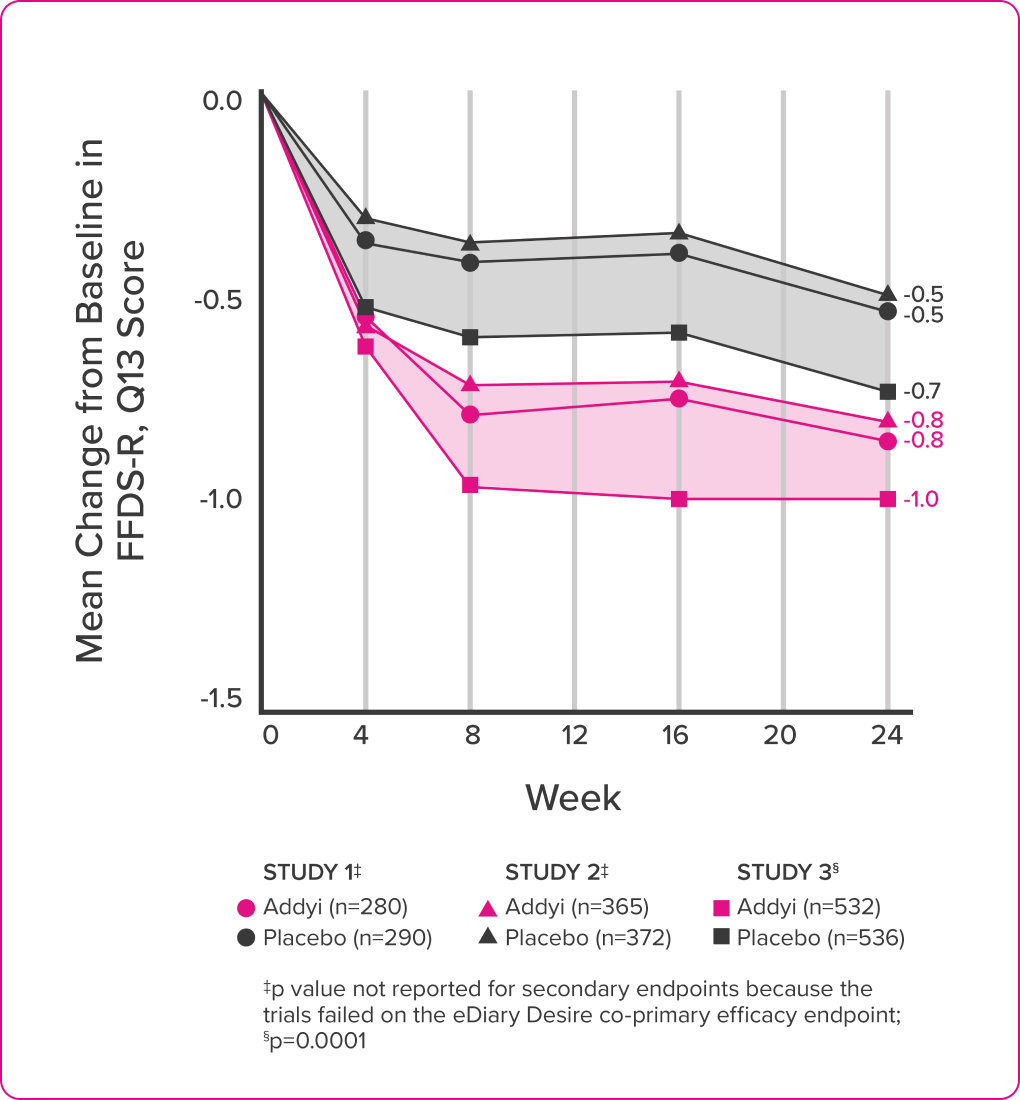
In clinical trials, all three endpoints were met.



Clinical Trial Safety Profile
Clinical Trial Safety Profile
Adverse Reactions Leading to Discontinuation
The discontinuation rate due to adverse reactions was between 9% and 13% among postmenopausal and premenopausal patients treated with 100mg Addyi at bedtime and between 5% and 6% among patients treated with placebo. Adverse reactions** leading to discontinuation in 6 randomized, double-blind, placebo-controlled trials in women with HSDD (<65 Years of Age) are shown below.
** Adverse reactions leading to discontinuation of >1% of patients who received ADDYI 100 mg once daily at bedtime and at a higher incidence than placebo-treated patients in the pooled premenopausal women and postmenopausal women trials.
| Trials in Premenopausal Women | Trials in Postmenopausal Women | |||
|---|---|---|---|---|
| Adverse Reaction | PLACEBO (n=1556) | ADDYI (n=1543) | PLACEBO (n=797) | ADDYI (n=801) |
| Dizziness | 0.1% | 1.7% | 0.3% | 0.9% |
| Nausea | 0.1% | 1.2% | 0.3% | 0.5% |
| Insomnia | 0.2% | 1.1% | 0.5% | 1.4% |
| Somnolence | 0.3% | 1.1% | 0.1% | 0.6% |
| Anxiety | 0.3% | 1.0% | 0.6% | 1.2% |
Most Common Adverse Reactions
Common adverse reactions† in 6 randomized, double-blind, placebo-controlled trials in postmenopausal and premenopausal women with HSDD (<65 Years of Age) are shown below. The majority of these adverse reactions began within the first 14 days of treatment.
† Adverse reactions reported in ≥2% of patients who received ADDYI 100 mg once daily at bedtime and at a higher incidence than placebo-treated patients in premenopausal women or postmenopausal women trials.
| Trials in Premenopausal Women | Trials in Postmenopausal Women | |||
|---|---|---|---|---|
| Adverse Reaction | PLACEBO (n=1556) | ADDYI (n=1543) | PLACEBO (n=797) | ADDYI (n=801) |
| Dizziness | 2.2% | 11.4% | 3.3% | 7.9% |
| Somnolence | 2.9% | 11.2% | 1.8% | 7.7% |
| Nausea | 3.9% | 10.4% | 3.9% | 6.6% |
| Fatigue | 5.5% | 9.2% | 3.9% | 3.0% |
| Insomnia | 2.8% | 4.9% | 3.4% | 5.7% |
| Dry Mouth | 1.0% | 2.4% | 1.3% | 2.4% |
| Urinary Tract Infection | 2.4% | 2.3% | 3.0% | 3.2% |
| Anxiety | 1.0% | 1.8% | 1.6% | 2.6% |
| Sinusitis | 3.5% | 2.9% | 2.1% | 2.5% |
| Constipation | 0.4% | 1.6% | 1.8% | 2.5% |
Post Hoc Analysis of Female Sexual Function Index (FSFI)
Additional Data
Post Hoc Analysis of Female Sexual Function Index (FSFI)
Additional Data
Post hoc analyses of FSFI total and individual domain data were pooled from 3 pivotal, multicenter, randomized, placebo-controlled, double-blind trials in premenopausal women with HSDD who received flibanserin (n=1165) or placebo (n=1203). Addyi has not been studied for the treatment of any female dysfunction other than acquired, generalized HSDD.
The Female Sexual Function Index (FSFI) is a 19-item patient-reported outcome tool measuring female sexual function. The tool has been validated for sexually active, heterosexual women and includes domains related to desire, arousal, lubrication, satisfaction and pain. The FSFI-6 is a 6-question survey intended to screen women at risk of female sexual dysfunction and includes questions on desire, arousal, lubrication, satisfaction, pain, and orgasm.
Although FSFI was evaluated in Addyi clinical trials, these clinical trials were not adequately powered to determine treatment effects on the individual components of the FSFI. These analyses were not prespecified and appropriate multiplicity adjustments were not applied. Results need cautious interpretation and no conclusions about treatment effect can be drawn.
Orgasm
Lubrication
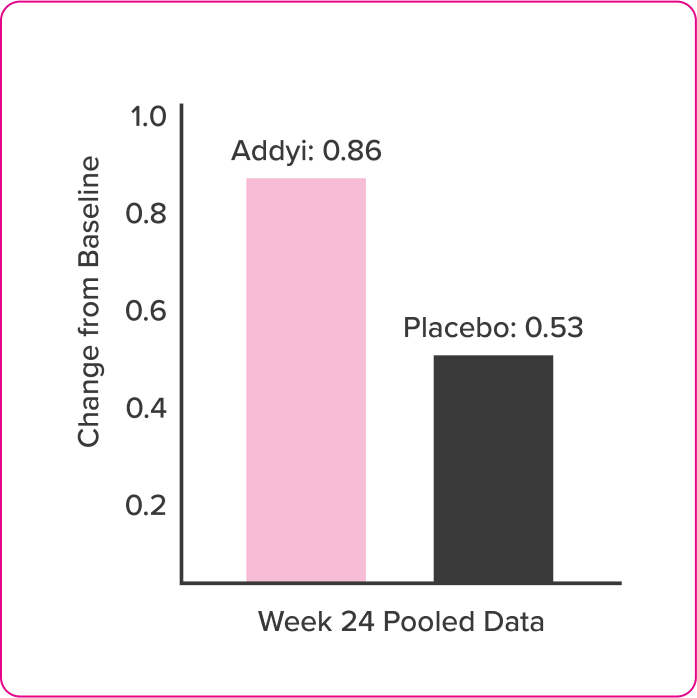
Arousal
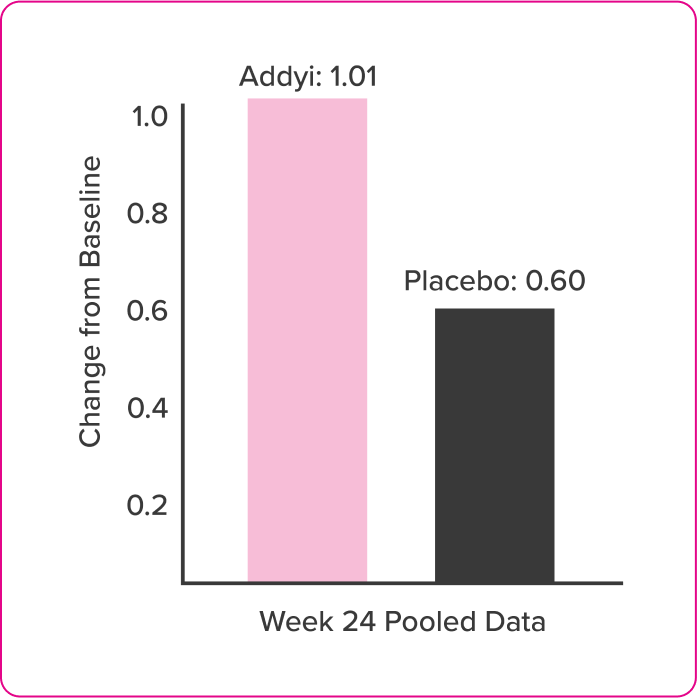
Desire
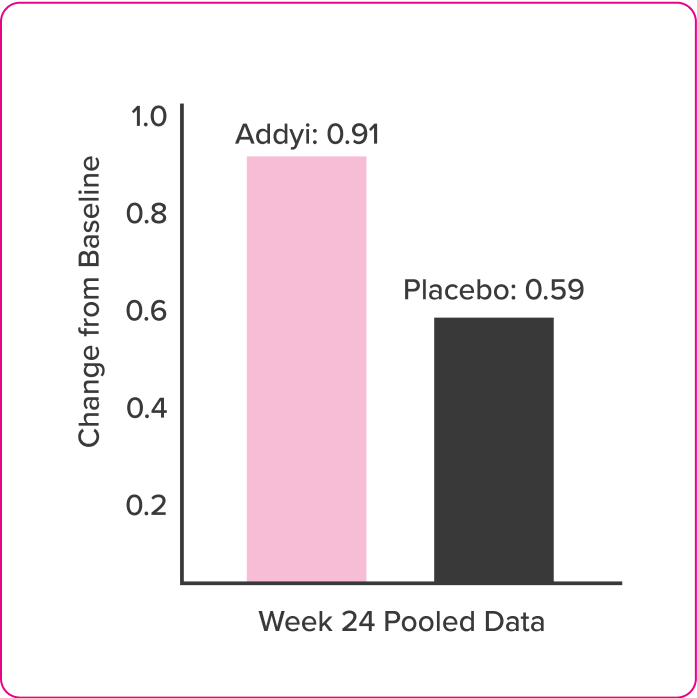
Satisfaction
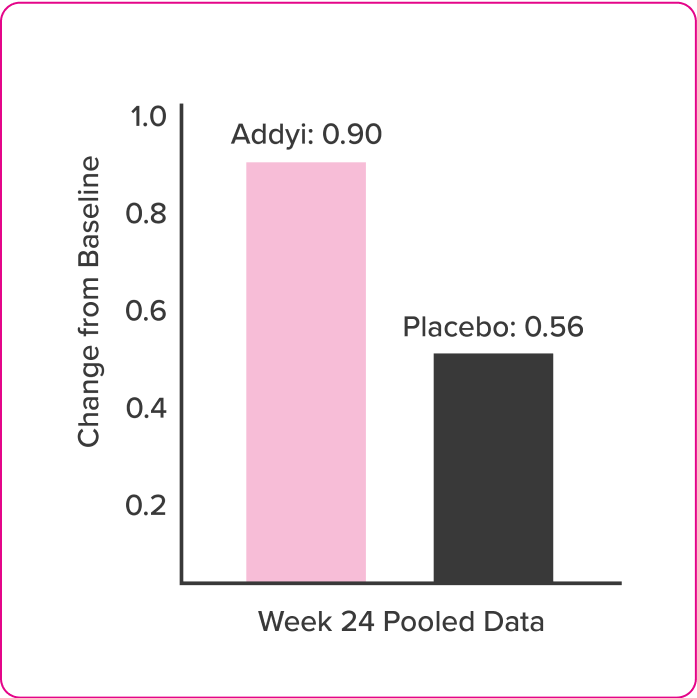
Pain Reduction
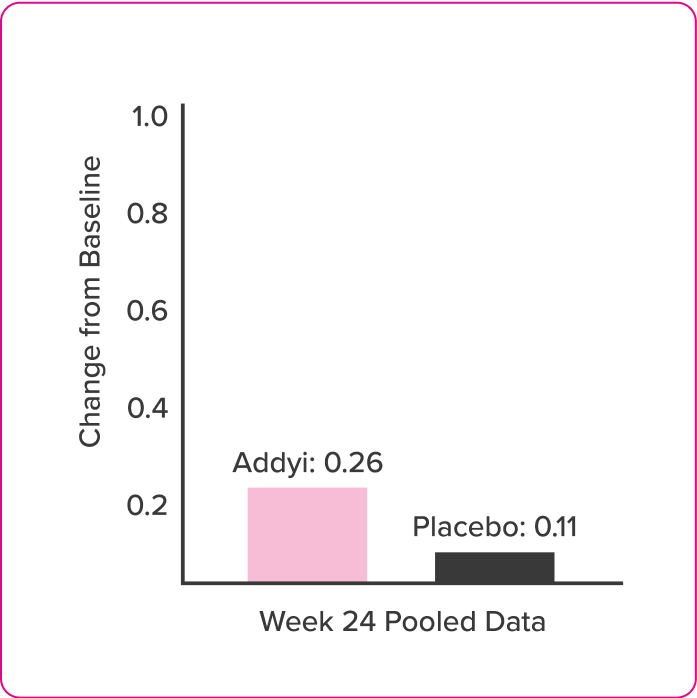
Orgasm

Lubrication

Arousal

Desire

Satisfaction

Pain Reduction

Post Hoc Analysis of FSFI Domain Scores
- Post-hoc exploratory analyses compared change from baseline in FSFI scores of flibanserin and placebo groups at each assessment time point by t-test
- FSFI questionnaire was administered at baseline and Weeks 4, 8, 16, and 24
- There was no adjustment for multiple comparisons
- Missing data were handled using the last observation carried forward (LOCF) method
‡Post hoc analysis sponsored by Sprout.
Post Hoc Analysis of Effect On Weight
Post hoc analysis of pooled data from 3 pivotal, multicenter, randomized, placebo-controlled, double-blind trials in premenopausal women with HSDD who received flibanserin. Addyi is not indicated for weight loss.
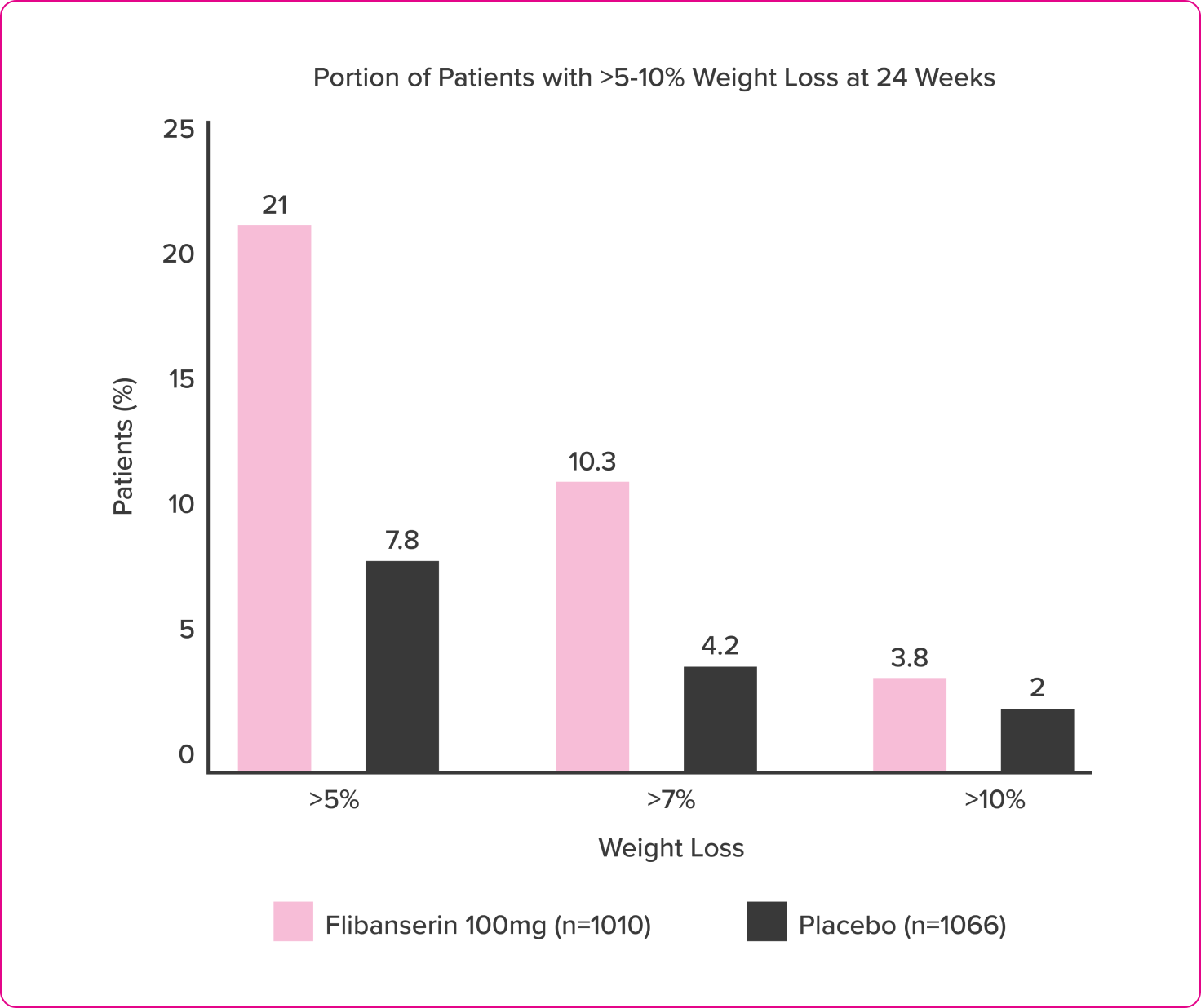
Post Hoc Analysis of Pooled Data from 3 Clinical Trials in Women with HSDD.
- Mean baseline weight was ~73kg (~160lbs)
- Weight gain ≥7% at 24 weeks occurred in 1.8% women receiving flibanserin and 3.4% women receiving placebo
- Higher baseline BMI was associated with greater weight loss.
- No association seen between effect on weight and treatment response, contraceptive use, smoking status, SSRI/SNRI use, or occurrence of nausea
- Body weight was measured to assess weight loss and weight gain as potential adverse events
- Study was not designed to evaluate weight loss. Patients were not selected based on obesity status nor did they enter the studies with the goal of losing weight.
Post Hoc Analysis of Effect On Weight
Post hoc analysis of pooled data from 3 pivotal, multicenter, randomized, placebo-controlled, double-blind trials in premenopausal women with HSDD who received flibanserin. Addyi is not indicated for weight loss.

Post Hoc Analysis of Pooled Data from 3 Clinical Trials in Women with HSDD.
- Mean baseline weight was ~73kg (~160lbs)
- Weight gain ≥7% at 24 weeks occurred in 1.8% women receiving flibanserin and 3.4% women receiving placebo
- Higher baseline BMI was associated with greater weight loss.
- No association seen between effect on weight and treatment response, contraceptive use, smoking status, SSRI/SNRI use, or occurrence of nausea
- Body weight was measured to assess weight loss and weight gain as potential adverse events
- Study was not designed to evaluate weight loss. Patients were not selected based on obesity status nor did they enter the studies with the goal of losing weight.
Coadministration with SSRI/SNRI
Clinical considerations for taking Addyi with SSRIs and SNRIs
Coadministration with SSRI/SNRI
Clinical considerations for taking Addyi with SSRIs and SNRIs
| Fluvoxamine (LuvoxⓇ) |
|
||
|---|---|---|---|
| Citalopram (CelexaⓇ) Escitalpram (LexaproⓇ) Fluoxetine (ProzacⓇ) Paroxetine (PaxilⓇ) Sertaline (ZoloftⓇ) Vilazodone (ViibrydⓇ) Desvenlafaxine (PristiqⓇ) Duloxetine (CymbaltaⓇ) Levomilnacipran (FetzimaⓇ) Milnasipran (SavellaⓇ) Venlafaxine (EffexorⓇ) |
|
Information based on pharmacologic action of products based on respective Prescribing Information as of January 2025. All product names, trademarks and registered trademarks are property of their respective owners.
Coadministration with SSRI/SNRI9
12 week randomized, double-blind, placebo-controlled clinical trial in 111 premenopausal women with mild to remitted depression treated with a stable dose of SSRI/SNRI* and symptoms of HSDD.**
| RESULT | Filbanserin† + SSRI/SNRI (%) N=72 | Placebo + SSRI/SNRI (%) N=37 | |
|---|---|---|---|
| Primary Endpoint Incidence AEs | 65.8 | 71.1 | |
| Depression (QIDS-SR16) | Remission No change Worsened |
19.4 73.6 6.9 |
10.8 67.6 21.6 |
| Anxiety (Beck Anxiety Inventory) | Remission No change Worsened |
16.4 82.2 1.4 |
2.7 94.6 2.7 |
- Overall, no increased risk of adverse events, including depression and anxiety were observed
- No instances of suicidality (C-SSRS)
- AEs ≥3% with Addyi and higher than placebo: dry mouth, insomnia, back pain, dizziness
- This study was designed to assess flibanserin safety; No conclusions regarding efficacy can be made
*citalopram, escitalopram, fluoxetine, paroxetine, sertraline, desvenlafaxine, duloxetine, venlafaxine **Planned sample size was 200 patients; study was terminated early due to commercial reasons †Includes 28 patients on fixed 100 mg qhs dose and 45 patients on up-titrated dose (50 mg qhs first two weeks, followed by 100 mg qhs). C-SSRS = Columbia-Suicide Severity Rating Scale; QIDS-SR16 = 16-item Quick Inventory of Depressive Symptomology-Self Report
Coadministration with SSRI/SNRI9
12 week randomized, double-blind, placebo-controlled clinical trial in 111 premenopausal women with mild to remitted depression treated with a stable dose of SSRI/SNRI* and symptoms of HSDD.**
| RESULT | Filbanserin† + SSRI/SNRI (%) N=72 | Placebo + SSRI/SNRI (%) N=37 | |
|---|---|---|---|
| Primary Endpoint Incidence AEs | 65.8 | 71.1 | |
| Depression (QIDS-SR16) | Remission No change Worsened |
19.4 73.6 6.9 |
10.8 67.6 21.6 |
| Anxiety (Beck Anxiety Inventory) | Remission No change Worsened |
16.4 82.2 1.4 |
2.7 94.6 2.7 |
- Overall, no increased risk of adverse events, including depression and anxiety were observed
- No instances of suicidality (C-SSRS)
- AEs ≥3% with Addyi and higher than placebo: dry mouth, insomnia, back pain, dizziness
- This study was designed to assess flibanserin safety; No conclusions regarding efficacy can be made
*citalopram, escitalopram, fluoxetine, paroxetine, sertraline, desvenlafaxine, duloxetine, venlafaxine **Planned sample size was 200 patients; study was terminated early due to commercial reasons †Includes 28 patients on fixed 100 mg qhs dose and 45 patients on up-titrated dose (50 mg qhs first two weeks, followed by 100 mg qhs). C-SSRS = Columbia-Suicide Severity Rating Scale; QIDS-SR16 = 16-item Quick Inventory of Depressive Symptomology-Self Report
Clinically Significant Drug Interactions with Addyi7
Clinically Significant Drug Interactions with Addyi7
| Alcohol | |
|---|---|
| Clinical Implications | The coadministration of ADDYI with alcohol increased the risk of hypotension syncope, and CNS depression compared to the use of ADDYI alone or alcohol alone. |
| Preventing or Managing DI | Counsel patients to wait at least two hours after consuming one or two standard alcoholic drinks before taking ADDYI at bedtime or to skip their ADDYI dose if they have consumed three or more alcoholic drinks that evening. |
| Other CNS Depressants | Diphenhydramine, opioids, hypnotics, benzodiazepines |
| Clinical Implications | The concomitant use of ADDYI with CNS depressants may increase the risk of CNS depression (e.g., somnolence) compared to the use of ADDYI alone. |
| Preventing or Managing DI | Discuss the concomitant use of other CNS depressants with the patient when prescribing ADDYI. |
| Moderate or Strong CYP3A4 Inhibitors | Strong: Oral contraceptives, cimetidine, fluxetine, ginkgo, ranitidine Moderate: Amprenavir, atazanavir, ciprofloxacin, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, and grapefruit juice |
| Clinical Implications | The concomitant use of ADDYI with moderate or strong CYP3A4 inhibitors increases flibanserin exposure compared to the use of ADDYI alone. The risk of hypotension and syncope is increased with concomitant use of ADDYI and moderate or strong CYP3A4 inhibitors. |
| Preventing or Managing DI | The concomitant use of ADDYI with moderate or strong CYP3A4 inhibitors is contraindicated. |
| Weak CYP3A4 Inhibitors | Oral contraceptives, cimetidine, fluxetine, ginkgo, ranitidine |
| Clinical Implications | The concomitant use of ADDYI with multiple weak CYP3A4 inhibitors may increase the risk of adverse reactions. |
| Preventing or Managing DI | Discuss the use of multiple weak CYP3A4 inhibitors with the patient when prescribing ADDYI. |
| Strong CYP2C19 Inhibitors | Proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals |
| Clinical Implications | The concomitant use of ADDYI with strong CYP2C19 inhibitors may increase flibanserin exposure which may increase the risk of hypotension, syncope, and CNS depression. |
| Preventing or Managing DI | Discuss the use of a strong CYP2C19 inhibitor with the patient when prescribing ADDYI. |
| CYP3A4 Inducers | Carbamazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapetine, St. John’s Wort |
| Clinical Implications | The concomitant use of ADDYI with CYP3A4 inducers substantially decreases flibanserin exposure compared to the use of ADDYI alone. |
| Preventing or Managing DI | The concomitant use of ADDYI with CYP3A4 inducers is not recommended. |
| Digoxin or Other P-glycoprotein Substrates | Digoxin, Sirolimus |
| Clinical Implications | The concomitant use of ADDYI with digoxin, a drug that is transported by P-glycoprotein (P-gp), increases the digoxin concentration. This may lead to digoxin toxicity. |
| Preventing or Managing DI | Increase monitoring of concentrations of drugs transported by P-gp that have a narrow therapeutic index (e.g., digoxin). |
Contact Us
MEDICAL INFO:
844-746-5745 x 3
medicalaffairs@sproutpharma.com
Contact Us
MEDICAL INFO:
844-746-5745 x 3
medicalaffairs@sproutpharma.com
- IQVIA Monthly Total Prescriptions Volume Data Comparing Addyi vs Vyleesi in the US. Sept 2024–Dec 2025
- Ryan KL, Arbuckle-Bernstein V, Smith G, Phillips J. Let’s Talk About Sex: A Survey of Patients’ Preferences When Addressing Sexual Health Concerns in a Family Medicine Residency Program Office. PRiMER. 2018;2:23. https://doi.org/10.22454/PRiMER.2018.728252
- Stahl SM, et al. Multifunctional pharmacology of flibanserin: possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011;8:15-27
- Www.medicalnewstoday.com, 30 Nov. 2022, www.medicalnewstoday.com/articles/hypoactive-sexual-desire-disorder#overview
- Gerstenberger EP, Rosen RC, Brewer JV, et al. Sexual desire and the Female Sexual Function Index (FSFI): a sexual desire cutpoint for clinical interpretation of the FSFI in women with and without hypoactive sexual desire disorder. J Sex Med. 2010;7(9):3096–3103.
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2006; 26:2, 191-208.
- Addyi Prescribing Information.
- https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers
- Clayton AH, Harry AC, Yuan J, et al. Safety of flibanserin in women treated with antidepressants: A randomized, placebo-controlled study. J Sex Med 2018;15(1):43-51.
- Arnow BA, Millheiser L, Garrett A et al. Women with hypoactive sexual desire disorder compared to normal females: a functional magnetic resonance imaging study. Neuroscience 2009;158:484-502.
- Woodard TL, Nowak NT, Balon R et al. Brain activation patterns in women with acquired hypoactive sexual desire disorder and women with normal sexual function: a cross-sectional pilot study. Fertil Steril 2013;100:1068-1076.
- Bianchi-Demicheli F, Cojan Y, Waber L, et al. Neural basis of hypoactive sexual desire disorder in women: an event-related fmri study. J Sex Med. 2011;8:2546-2559.
- Holstege G. How the emotional motor system controls the pelvic organs. Sex Med Rev. 2016: 4;303-328.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Women’s Health. 2014;23(10):817-23
- Clayton AH, Brown L, Kim NN. Evaluation of safety for flibanserin. Expert Opin Drug Saf. 2020;19(1):1-8. doi:10.1080/14740338.2020.1707804
- Goldfischer ER, Breaux J, Katz M et al. Continued efficacy and safety of flibanserin in premenopausal women with hypoactive sexual desire disorder (HSDD): Results from a randomized withdrawal trial. J Sex Med.2011; 8:3160-3172.
- Jayne C, Simon JA, Taylor LV, Kimura T, Lesko LM; SUNFLOWER study investigators. Open-label extension study of flibanserin in women with hypoactive sexual desire disorder. J Sex Med. 2012;9(12):3180-3188. doi:10.1111/j.1743-6109.2012.02942.x
- Kornstein SG, James JA, Apfel SC, et al. Effect of flibanserin treatment on body weight in premenopausal and postmenopausal women with hypoactive sexual desire disorder: A post hoc analysis. J Women’s Health. 2017;26(11):1161-1168.
INDICATION AND IMPORTANT SAFETY INFORMATION INCLUDING BOXED WARNING
WARNING: HYPOTENSION AND SYNCOPE IN CERTAIN SETTINGS
See full prescribing information for complete boxed warning.
- Use of ADDYI and alcohol together close in time increases the risk of severe hypotension and syncope. Counsel patients to wait at least two hours after consuming one or two standard alcoholic drinks before taking ADDYI at bedtime or to skip their ADDYI dose if they have consumed three or more standard alcoholic drinks that evening.
- Severe hypotension and syncope can occur when ADDYI is used with moderate or strong CYP3A4 inhibitors or in patients with hepatic impairment; therefore, ADDYI use in these settings is contraindicated.
INDICATION
ADDYI (flibanserin) is indicated for the treatment of women less than 65 years of age with acquired, generalized hypoactive sexual desire disorder (HSDD) as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- A co-existing medical or psychiatric condition,
- Problems within the relationship, or
- The effects of a medication or other drug substance.
Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation, or partner.
Limitations of Use:
- ADDYI is not indicated in men.
- ADDYI is not indicated to enhance sexual performance.
Contraindications
- Moderate or strong cytochrome P450 3A4 (CYP3A4) inhibitors
- Hepatic impairment
- Known hypersensitivity to ADDYI or any of its components. Reactions, including anaphylaxis, reactions consistent with angioedema, pruritus, and urticaria have been reported.
Warnings and Precautions
- Hypotension and Syncope Due to an Interaction with Alcohol: See Boxed Warning for risks associated with alcohol consumption. After taking ADDYI at bedtime, advise patients to not use alcohol until the following day.
- Hypotension and Syncope with CYP3A4 Inhibitors: See Boxed Warning and Contraindication about use with moderate or strong cytochrome P450 3A4. Concomitant use of multiple weak CYP3A4 inhibitors that may include herbal supplements (e.g., ginkgo, resveratrol) or non-prescription drugs (e.g., cimetidine) could also lead to clinically relevant increases in flibanserin concentrations that may increase the risk of hypotension and syncope.
- Central Nervous System (CNS) Depression (e.g., Somnolence, Sedation): Can occur with ADDYI alone and is exacerbated by other CNS depressants. The risk of CNS depression is also increased if ADDYI is taken during waking hours. Patients should avoid activities requiring full alertness (e.g., operating machinery or driving) until at least six hours after each dose and until they know how ADDYI affects them.
- Hypotension and Syncope with ADDYI Alone: The use of ADDYI - without other concomitant medications known to cause hypotension or syncope - can cause hypotension and syncope. The risk is increased if ADDYI is taken during waking hours or if higher than the recommend dose is taken.
- Syncope and Hypotension in Patients with Hepatic Impairment: Any degree of hepatic impairment significantly increases flibanserin concentrations, which can lead to hypotension and syncope. ADDYI is contraindicated in patients with hepatic impairment.
- Hypersensitivity Reactions: Reactions including anaphylaxis, reactions consistent with angioedema, pruritus, and urticaria have been reported with ADDYI. Immediately discontinue ADDYI and initiate appropriate treatment if hypersensitivity reaction occurs.
Drug Interactions
- Oral Contraceptives and Other Weak CYP3A4 Inhibitors: Increases flibanserin exposures and incidence of adverse reactions.
- Strong CYP2C19 Inhibitors: Increases flibanserin exposure which may increase risk of hypotension, syncope, and CNS depression.
- CYP3A4 Inducers: Use of ADDYI not recommended, flibanserin concentrations substantially reduced
- Digoxin: Increases digoxin concentrations, which may lead to digoxin toxicity. Increase monitoring of digoxin concentrations.
Most Common Adverse Reactions
- Most common adverse reactions (ADDYI incidence ≥2% and higher than placebo) are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
See Full Prescribing Information and Medication Guide, including Boxed Warning regarding hypotension and syncope in certain settings.
INDICATION
ADDYI (flibanserin) is indicated for the treatment of women less than 65 years of age with acquired, generalized hypoactive sexual desire disorder (HSDD) as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- A co-existing medical or psychiatric condition,
- Problems within the relationship, or
- The effects of a medication or other drug substance.
Acquired HSDD refers to HSDD that develops in a patient who previously had no problem with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation, or partner.
Limitations of Use:
- ADDYI is not indicated for use in men.
- ADDYI is not indicated to enhance sexual performance.
WARNING: HYPOTENSION AND SYNCOPE IN CERTAIN SETTINGS
See full prescribing information for complete boxed warning.
- Use of ADDYI and alcohol together close in time increases the risk of severe hypotension and syncope. Counsel patients to wait at least two hours after consuming one or two standard alcoholic drinks before taking ADDYI at bedtime or to skip their ADDYI dose if they have consumed three or more standard alcoholic drinks that evening.
- Severe hypotension and syncope can occur when ADDYI is used with moderate or strong CYP3A4 inhibitors or in patients with hepatic impairment; therefore, ADDYI use in these settings is contraindicated.
Contraindications
- Moderate or strong cytochrome P450 3A4 (CYP3A4) inhibitors
- Hepatic impairment
- Known hypersensitivity to ADDYI or any of its components. Reactions, including anaphylaxis, reactions consistent with angioedema, pruritus, and urticaria have been reported.
Warnings and Precautions
- Hypotension and Syncope Due to an Interaction with Alcohol: See Boxed Warning for risks associated with alcohol consumption. After taking ADDYI at bedtime, advise patients to not use alcohol until the following day.
- Hypotension and Syncope with CYP3A4 Inhibitors: See Boxed Warning and Contraindication about use with moderate or strong cytochrome P450 3A4. Concomitant use of multiple weak CYP3A4 inhibitors that may include herbal supplements (e.g., ginkgo, resveratrol) or non-prescription drugs (e.g., cimetidine) could also lead to clinically relevant increases in flibanserin concentrations that may increase the risk of hypotension and syncope.
- Central Nervous System (CNS) Depression (e.g., Somnolence, Sedation): Can occur with ADDYI alone and is exacerbated by other CNS depressants. The risk of CNS depression is also increased if ADDYI is taken during waking hours. Patients should avoid activities requiring full alertness (e.g., operating machinery or driving) until at least six hours after each dose and until they know how ADDYI affects them.
- Hypotension and Syncope with ADDYI Alone: The use of ADDYI - without other concomitant medications known to cause hypotension or syncope - can cause hypotension and syncope. The risk is increased if ADDYI is taken during waking hours or if higher than the recommend dose is taken.
- Syncope and Hypotension in Patients with Hepatic Impairment: Any degree of hepatic impairment significantly increases flibanserin concentrations, which can lead to hypotension and syncope. ADDYI is contraindicated in patients with hepatic impairment.
- Hypersensitivity Reactions: Reactions including anaphylaxis, reactions consistent with angioedema, pruritus, and urticaria have been reported with ADDYI. Immediately discontinue ADDYI and initiate appropriate treatment if hypersensitivity reaction occurs.
Drug Interactions
- Oral Contraceptives and Other Weak CYP3A4 Inhibitors: Increases flibanserin exposures and incidence of adverse reactions.
- Strong CYP2C19 Inhibitors: Increases flibanserin exposure which may increase risk of hypotension, syncope, and CNS depression.
- CYP3A4 Inducers: Use of ADDYI not recommended, flibanserin concentrations substantially reduced
- Digoxin: Increases digoxin concentrations, which may lead to digoxin toxicity. Increase monitoring of digoxin concentrations.
Most Common Adverse Reactions
- Most common adverse reactions (ADDYI incidence ≥2% and higher than placebo) are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
See Full Prescribing Information and Medication Guide, including Boxed Warning regarding hypotension and syncope in certain settings.
